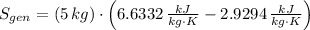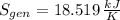Answer:
Step-by-step explanation:
Given that rigid tank is a closed system, the following model is constructed after the First Law of Thermodynamics:
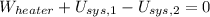
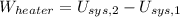
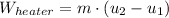
The entropy generation inside the rigid tank is determined by appropriate application of the Second Law of Thermodynamics:
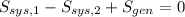
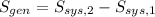
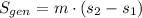
The properties of the steam are obtained from steam tables:
Intial State
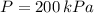
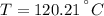
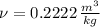
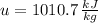
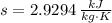
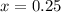
Final State
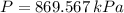
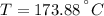
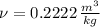
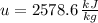
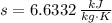
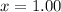
The entropy change of the steam during the process is: