Answer:
-165 (without units).
Step-by-step explanation:
Hello,
In this case, in terms of the first law of thermodynamics:
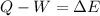
Taking into account the 403 J of work done by the system and the 238 of heat absorbed from the surroundings, the change in energy for the gas results:
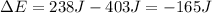
Thus, the value without units is -165.
Regards.