Answer:
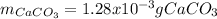
Step-by-step explanation:
Hello,
In this case, for the first titration, the molarity of the MnSO₄ is:
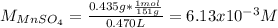
Now, the moles that are neutralized by the EDTA for the 45.50-mL aliquot are:
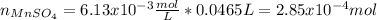
In such a way, those moles equals the EDTA moles based on the titration main equation:
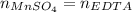
Next, the concentration of the EDTA 41.9-mL solution is:
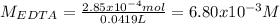
Afterwards, for the second titration, the moles of EDTA that equal the neutralized grams of calcium carbonate are:
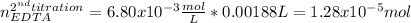
Finally, the grams of calcium carbonate turn out:
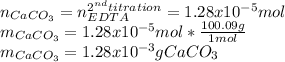
Best regards.