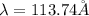Answer:
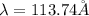
Step-by-step explanation:
Electron is jumping from n=1 to n=3
and for Li Z=3;
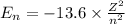
![\Delta E=-13.6* Z^2[(1)/(n_3^2) -(1)/(n_1^2)]](https://img.qammunity.org/2021/formulas/chemistry/college/7mdsyvna3z4j07b9kwuooxvuvv5sx5lr20.png)
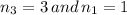
after solving we get;
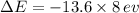
for the photon energy is calculated by the following equation
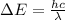
for easy calculation;
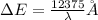
where energy is taken in electron volt
after puting value of
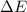 we get,
we get,