Answer:
Na = 9.25 x 10⁻⁷
Step-by-step explanation:
ρ1 = 1003 kg/m³
ρ2 = 1012 kg/m³
Dab = 0.95 x 10⁻⁹ m²/s
Z = 10⁻³
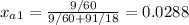
xa2 = (3/18) / (3/18 + 97/60) = 0.0092
Mavg1 = (9/100 * 60) + (91/100 * 18) = 21.78
Mavg2 = (3/100 * 18) + (97/100 * 60) = 19.26
ρavg = (ρ1 + ρ2) / 2 = (1003 + 1012) / 2 =1007.5 kg/m³
(ρ/M)avg = (ρavg/Mavg1 + ρavg/Mavg2) / 2 = (46.25 + 52.31) / 2 = 49.28
Na = -(ρ/M)avg * Dab / Z * ln [(1-xa1) / (1 - xa2)]
= -49.28 * 0.95 x 10⁻⁹ / 10⁻³ * ln [(1-0.0288) / (1 - 0.0092)] = 9.25 x 10⁻⁷