Answer:
The work required is W = 20.2 BTU per lbm
Step-by-step explanation:
The value of entropy & enthalpy at initial conditions are
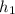 = 103.1
= 103.1
S = 0.225
Final enthalpy
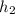 = 123.3
= 123.3
Therefore work done
W =
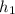 -
-
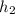
W = 103.1 - 123.3
W = - 20.2 BTU per lbm
Therefore the work required is W = 20.2 BTU per lbm