Answer:
The energy used to reach the surface is 1.53 eV.
Step-by-step explanation:
Given that, the work function of a certain metal is 6.6 eV.
Frequency of photon,
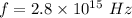
Kinetic energy of the electron,
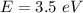
The energy of the photon is:
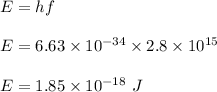
Energy utilized by the photon is given by :
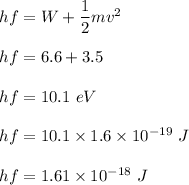
Used energy is given by the difference of energy given and the energy utilized. So,
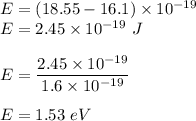
So, energy used to reach the surface is 1.53 eV.