Answer:
Step-by-step explanation:
First we find the Molecular weight of CaCl2
looking at the periodic table the MW of Ca is 40.078 and Cl is 35.453 and the ratio of ca to cl is 1:2 so we multiply cl by 2 which is 35.453*2=70.906
we add them together: 40.078 + 70.906 = 110.984 g/mol
now:
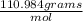 * 3.45 mol
* 3.45 mol
the mol cancels out and we left with 3.45 * 110.984 grams = 382.89 grams