8.856 grams of water is needed by the plant to make 14.9 grams of glucose.
Step-by-step explanation:
Data given
mass of glucose needed by plant = 14.9 grams
mass of water needed =?
balanced chemical equation:
6C
 + 6
+ 6

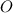 ⇒
⇒
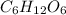 + 6
+ 6

molar mass of glucose = 180.15 grams/mole
number of moles =

number of moles =
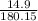
number of moles= 0.082 moles
from the equation:
1 mole of glucose is produced by 6 moles of water
0.082 moles of glucose is produced by x mole of water
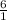 =
=
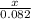
x = 0.492 moles of water
atomic mass of water = 18 grams/mole
mass of water required = number of moles x atomic mass of water
mass of water = 0.492 x 18
= 8.856 grams