Answer:
The solubility product of lead(II) chloride is
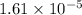 .
.
Step-by-step explanation:
Concentration of lead (II) ions =
![[Pb^(2+)]=0.0159 M](https://img.qammunity.org/2021/formulas/chemistry/college/r0s6qvouqtvzxcg56754yjtpcnv7srct4m.png)
Concentration of chloride ion =
![[Cl^-]=0.0318 M](https://img.qammunity.org/2021/formulas/chemistry/college/ve5typmmi138gbea7tlkfqvo3vs7cxqd8y.png)
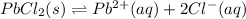
The expression of a solubility product will be given as:
![K_(sp)=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2021/formulas/chemistry/college/bnzapmsqdui2w7g2cdfebjt14frtn40x1e.png)
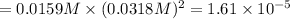
The solubility product of lead(II) chloride is
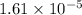 .
.