Answer:
Only one seven carbon hydrocarbon was produced.
Step-by-step explanation:
- Alkynes are reduced completely in presence of
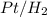 to produce alkanes.
to produce alkanes. - Hydrogenation in presence of Pt is a simple nucleophilic addition process where
 molecule adds onto an unsaturated bond.
molecule adds onto an unsaturated bond. - Hept-1-yne, hept-2-yne and hept-3-yne are constitutionally isomeric to each other. Hence, after complete reduction, all three alkynes produced heptane as only product.
- So, only one seven carbon hydrocarbon was produced.