Answer:
2080 kJ/mol is the first ionization of 1st atom and 496 kJ/mol is the first ionization of 2nd atom
Step-by-step explanation:
Given electronic configurations are :
1st:
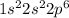
2nd :
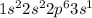
given 1st ionization energy are: 2080 kJ/mol and 496 kJ/mol
generally ionization energy of fulfilled orbital is more than half filled orbital and these two state are more stable.
therefore ionization energy of fulfilled is more than half filled orbital
hence
ionization energy of 1st atom will be very high because its orbital is fulfilled and less energy for 2nd atom so 2080 kJ/mol is the first ionization of 1st atom and 496 kJ/mol is the first ionization of 2nd atom.