Answer:
Step-by-step explanation:
Using your hint, the density of gases equation is:
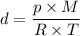
Where:
- d = density of the gas
- M = molar mass of the gas
- R = universal constant of gases
- T = absolute temperature
The conditions of your gas are:
State 1:
- d₁ = 0.87g/L
- T₁ = 30 + 273.15K = 303.15K
- p₁ = 131.2kPa
State 2 (STP):
- d₂ = ?
- T₂ = 0 + 273.15K = 273.15K
- p₂ = 100kPa (in the old definition of STP it was 101.325kPa)
Since M and R are constants, the density of gases equation drives to:
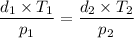
Now you can substitute and clear d₂
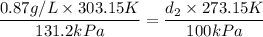
If you use 101.325kPa instead of 100kPa it will result 0.75g/L, which is the option C.