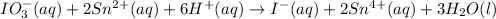Answer : The balanced chemical equation in acidic medium will be,
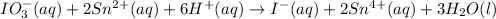
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
Rules for the balanced chemical equation in acidic solution are :
First we have to write into the two half-reactions.
Now balance the main atoms in the reaction.
Now balance the hydrogen and oxygen atoms on both the sides of the reaction.
If the oxygen atoms are not balanced on both the sides then adding water molecules at that side where the less number of oxygen are present.
If the hydrogen atoms are not balanced on both the sides then adding hydrogen ion
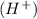 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.
Now balance the charge.
The given chemical reaction is,
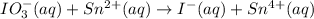
The oxidation-reduction half reaction will be :
Oxidation :
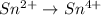
Reduction :
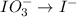
First balance the main element in the reaction.
Oxidation :
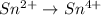
Reduction :
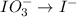
Now balance oxygen atom on both side.
Oxidation :
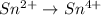
Reduction :
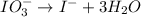
Now balance hydrogen atom on both side.
Oxidation :
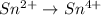
Reduction :
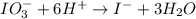
Now balance the charge.
Oxidation :
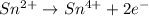
Reduction :
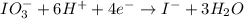
The charges are not balanced on both side of the reaction. Thus, we are multiplying oxidation reaction by 2 and the adding both equation, we get the balanced redox reaction.
Oxidation :
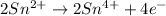
Reduction :
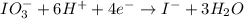
The balanced chemical equation in acidic medium will be,