Answer:
1)
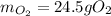
2)
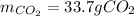
Step-by-step explanation:
Hello,
1) In this case, by considering the given reaction, starting by 23.0 g of glucose, the grams of oxygen by stoichiometry result:
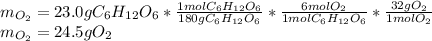
2), Now, by means of also stoichiometry, the grams of carbon dioxide that are produced result:
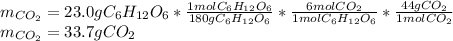
Best regards.