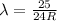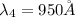Answer:
Wavelength of the first four spectral line:
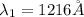
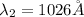
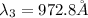
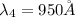
Step-by-step explanation:
Lman series when electron from higher shell number and jumps to 1st shell then all the possible lines is called as lyman series.
to calculate wavelength of first four spectral line:
For hydrogen Z=1;
by using rydberg equation
![(1)/(\lambda) =RZ^2[(1)/(n_1^2) -(1)/(n_2^2) ]](https://img.qammunity.org/2021/formulas/chemistry/college/nucv1kx46y2bm34b4tyoeoo2n8wcx2euia.png)
1. n=2 to n=1
![(1)/(\lambda) =RZ^2[(1)/(n_1^2) -(1)/(n_2^2) ]](https://img.qammunity.org/2021/formulas/chemistry/college/nucv1kx46y2bm34b4tyoeoo2n8wcx2euia.png)
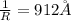 =rydberg constant
=rydberg constant
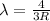
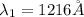
2. n=3 to n=1
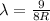
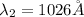
3. n=4 to n=1
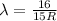
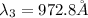
4. n=5 to n=1