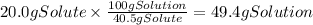Answer:
49.4 g Solution
Step-by-step explanation:
There is some info missing. I think this is the original question.
A chemistry student needs 20.0g of acetic acid for an experiment. He has 400.g available of a 40.5 % w/w solution of acetic acid in acetone.
Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to 3 significant digits.
We have 400 g of solution and there are 40.5 g of solute (acetic acid) per 100 grams of solution. We can use this info to find the mass of acetic acid in the solution.
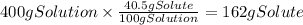
Since we only need 20.0 g of acetic acid, there is enough of it in the solution. The mass of solution that contains 20.0 g of solute is: