Answer: Its average atomic mass is 114.9 amu
Step-by-step explanation:
Mass of isotope 1 = 113 amu
% abundance of isotope 1 = 5% =
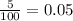
Mass of isotope 2 = 115 amu
% abundance of isotope 2 = 95% =
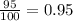
Formula used for average atomic mass of an element :
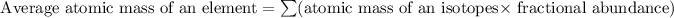
![A=\sum[(113* 0.05)+(115* 0.95)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/5ae2uqourknz7dwsx7qwojoyizkyv343qu.png)
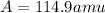
Thus its average atomic mass is 114.9 amu