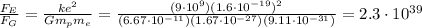1) No
2) Yes
3) No
4) Equal and opposite
5) 32400 N
6) Repulsive
7) The electric force is
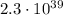 times bigger than the gravitational force
times bigger than the gravitational force
Step-by-step explanation:
1)
In nature, the minimum possible charge that an object can have is the charge of the electron, which is called fundamental charge:
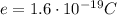
Electrons are indivisible particles (they cannot be separated), this means that an object can have at least the charge equal to the charge of one electron (in fact, it cannot have a charge less than
 , because it would meant that the object has a "fractional number" of electrons).
, because it would meant that the object has a "fractional number" of electrons).
In this problem, the object has a charge of
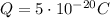
If we compare this value to
 , we notice that
, we notice that
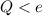 , so no object can have a charge of
, so no object can have a charge of
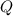 .
.
2)
As we said in part 1), an object should have an integer number of electrons in order to be charged.
This means that the charge of an object must be an integer multiple of the fundamental charge, so we can write it as:
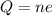
where
Q is the charge of the object
n is an integer multiple
e is the fundamental charge
Here we have
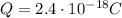
Substituting the value of e, we find n:
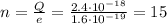
n is integer, so this value of the charge is possible.
3)
We now do the same procedure for the new object in this part, which has a charge of
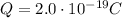
Again, the charge on this object can be written as
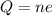
where
n is the number of electrons in the object
Using the value of the fundamental charge,
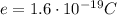
We find:
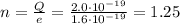
n is not integer, so this value of charge is not possible, since an object cannot have a fractional number of electrons.
4)
To solve this part, we use Newton's third law of motion, which states that:
"When an object A exerts a force on an object B (Action force), then object B exerts an equal and opposite force on object A (reaction force)".
In this problem, we have two objects:
- A charge Q
- A charge 5Q
Charge Q exerts an electric force on charge 5Q, and we can call this action force. At the same time, charge 5Q exerts an electric force on charge Q (reaction force), and according to Newton's 3rd law, the two forces are equal and opposite.
5)
The magnitude of the electric force between two single-point charges is
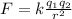
where
k is the Coulomb's constant
q1, q2 are the two charges
r is the separation between the two charges
In this problem we have:
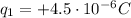 is charge 1
is charge 1
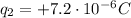 is charge 2
is charge 2
r = 0.30 cm = 0.003 m is the separation
So, the electric force between the two charges is
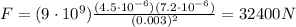
6)
The electric force between two charged objects has direction as follows:
- If the two objects have charges of opposite signs (+ and -), the force between them is attractive
- If the two objects have charges of same sign (++ or --), the force between them is repulsive
In this problem, the two charges are:
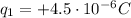 is charge 1
is charge 1
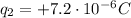 is charge 2
is charge 2
We see that the two charges have same sign: therefore, the force between them is repulsive.
7)
The electric force between the proton and the electron in the atom can be written as
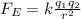
where
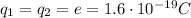 is the magnitude of the charge of the proton and of the electron
is the magnitude of the charge of the proton and of the electron
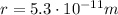 is the separation between them
is the separation between them
So the force can be rewritten as
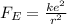
The gravitational force between the proton and the electron can be written as
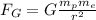
where
G is the gravitational constant
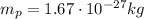 is the proton mass
is the proton mass
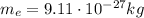 is the electron mass
is the electron mass
Comparing the 2 forces,