Answer:
Rate of disappearance of N₂O₅ = 17 x 10⁻³ M/sec
Step-by-step explanation:
Rate of reaction =
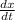
Where
dx = Change of concentration of reactants or products
dt = Change in time
According to chemical reaction
2 N₂O₅(g) → 4 NO₂(g)+O₂(g)
Rate of disappearance of N₂O₅ = Rate of appearance of O₂