Answer : The number of moles of
 added must be, 0.37 mol
added must be, 0.37 mol
Explanation :
The given chemical reaction is:
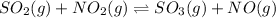
Initial mol. 2.99 x 0 0
At eqm. (2.99-1.30) (x-1.30) 1.30 1.30
= 1.69
The expression for equilibrium constant is:
![K_c=([SO_3][NO])/([SO_2][NO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/euu15xnqjw4x9pq5k7l6y6rzbweklg5k4s.png)
Now put all the given values in this expression, we get:
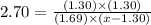
x = 1.67 mol
The moles of
 added = (x-1.30) = (1.67-1.30) = 0.37 mol
added = (x-1.30) = (1.67-1.30) = 0.37 mol