Answer:
The correct option is option (D).
Therefore the concentration of
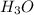 is 1.8×10⁻¹⁰ M and it is basic in nature.
is 1.8×10⁻¹⁰ M and it is basic in nature.
Step-by-step explanation:
List of pH:
- If pH of a solution is 7, then solution is neutral.
- If pH of a solution is grater than 7, then solution is basic.
- If pH of a solution is less than 7, then solution is acidic.
pH of a solution is = - log₁₀[H₃O⁺]
We know that,
[H₃O⁺][OH⁻] =
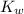 =
=
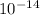 (at 25°C)
(at 25°C)
Taking log both sides
log ([H₃O⁺][OH⁻]) =log
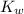 =log
=log
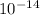
⇒log[H₃O⁺]+log[OH⁻] = log
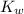 = -14 log 10
= -14 log 10
[since log(mn)= log m + log n,
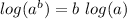 ]
]
⇒ - log[H₃O⁺] - log[OH⁻] = - (-14) [ log 10 =1]
⇒pH+pOH =14.
Given that,
The concentration of OH⁻ is 5.5 × 10⁻⁵ M
[H₃O⁺][OH⁻] =
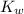 =
=
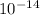
⇒[H₃O⁺] 5.5 × 10⁻⁵ M=10⁻¹⁴
![\Rightarrow [H_3O^+]=(10^(-14))/(5.5* 10^(-5))](https://img.qammunity.org/2021/formulas/chemistry/high-school/xloddbhy7h9ndiycv16hye67gtkhwe6ugy.png)
⇒[H₃O⁺] = 1.8×10⁻¹⁰ M
Now check the pH of the solution.
[H₃O⁺] = 1.8×10⁻¹⁰
Taking log both sides
log [H₃O⁺] =log( 1.8×10⁻¹⁰)
⇒ -log [H₃O⁺] = - log( 1.8×10⁻¹⁰)
⇒ pH = - log( 1.8×10⁻¹⁰)
⇒ pH =9.7.
So the nature of solution is basic.
Therefore the concentration of
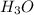 is 1.8×10⁻¹⁰ M and it is basic in nature.
is 1.8×10⁻¹⁰ M and it is basic in nature.