Answer:
The value of he change in Gibbs free energy ΔG = - 18.083 KJ
Step-by-step explanation:
Given data
The concentration of glucose inside a cell is (P) = 0.12 m M
The concentration of glucose outside a cell is (R) = 12.9 m M
No. of moles = 1.5 moles
The change in Gibbs free energy
ΔG = RT ㏑
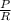
ΔG = 8.314 × 310 ㏑
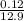
ΔG = - 12.055
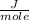
Since No. of moles = 1.5 moles
Therefore
ΔG = - 12.055 × 1.5
ΔG = - 18.083 KJ
This the value of he change in Gibbs free energy.