Answer: Temperature for the given reaction is 1040 K (approx).
Step-by-step explanation:
Formula for enthalpy change of a reaction is as follows.
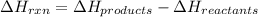
For the given reaction equation,
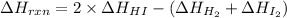
Now, putting the given values into the above formula as follows.
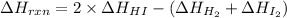
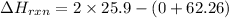
= -10460 J/mol
Now, we will calculate the change in its entropy as follows.
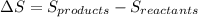
=
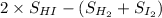
=
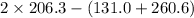
= 21 J/mol
Also, we know that
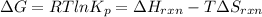
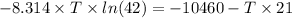
T = 1036.7 K
= 1040 K
Therefore, we can conclude that temperature for the given reaction is 1040 K (approx).