Answer:
3.
Step-by-step explanation:
Hello,
In this case, it is convenient to write the chemical reaction as:
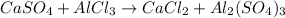
Which balanced turns out:
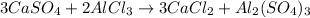
Thus the number that should be in front of the calcium sulfate is 3 in order to balance the reaction.
Best regards.