Answer:
Therefore water has its maximum density at 3.97°C.
Explanation:
Given that,
The volume of 1 kg of water at a temperature T is
V=999.87-0.06426T+0.0085043T²-0.0000679T³
where T is between 0°C and 30°C.
We know,
Density = Volume ÷ mass.
Here mass = 1 kg.
⇒Density = Volume ÷ 1 kg
⇒Density = Volume
Therefore the density of the water will be maximum only when the volume of the water is maximum.
V=999.87-0.06426T+0.0085043T²-0.0000679T³
Differentiating with respect T
V'= -0.06426+0.0170086T-0.0002037T²
Again differentiating with respect T
V''=0.0170086-0.0004074T
For the maximum value volume , V'=0
-0.06426+0.0170086T-0.0002037T²=0
⇒ 0.06426-0.0170086T+0.0002037T²=0
[ Applying quadratic formula
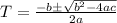 , here a=0.0002037, b =-0.0170086 and c =0.06426]
, here a=0.0002037, b =-0.0170086 and c =0.06426]
∴T=3.97, 79.53
Since the value of T is between 0°C and 30°C,
So, T=3.97°C
Therefore at T=3.97°C, the volume is maximum.
It means the density of the water will be maximum at 3.97°C.
Therefore water has its maximum density at 3.97°C.