Answer:
(a). Entropy change of refrigerant is = 0.7077
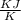
(b). Entropy change of cooled space
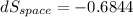
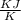
(c). Total entropy change is dS = 0.0232
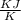
Step-by-step explanation:
Given data
Saturation pressure = 140 K pa
Saturation temperature from property table
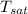 = - 18.77 °c = - 18.77 + 273 = 254.23 K
= - 18.77 °c = - 18.77 + 273 = 254.23 K
(a). Entropy change of refrigerant is given by
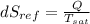
Since heat absorbed by refrigerant Q = 180 KJ
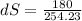
dS = 0.7077
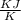
(b). Entropy change of cooled space
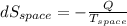
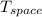 = - 10 °c = 263 K
= - 10 °c = 263 K
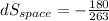
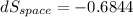
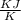
(c). Total entropy change is given by
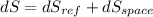
dS = 0.7077 - 0.6844
dS = 0.0232
This is the value of total entropy change.