Answer:
maximum amount of calcium carbonate=12.5 mili mole
Step-by-step explanation:
First write the balance chemical reaction:
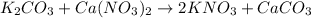
quantity of
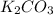 : 50ml and 0.25M
: 50ml and 0.25M
quantity of
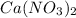 : 100ml and 0.175M
: 100ml and 0.175M
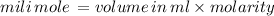
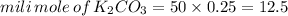
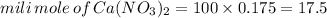
From the above balance equation it is clearly that:
1 mole of potassium carbonate react completely with 1 mole calcium nitrate
hence
12.5 mili mole of potassium carbonate react completely with 12.5 mili mole calcium nitrate but we have 17.5 mili mole of calcium nitrate therefore calcium nitrate will be the excess reactant and potassium carbonate will be the limiting reactant and quantiy of product depends on the limiting reactant i.e. potassium carbonate .
From the above balance equation:
1 mole of potassium carbonate gives 1 mole calcium carbonate
hence
12.5 mili mole will give 12.5 mili mole of calcium carbonate
maximum amount of calcium carbonate=12.5 mili mole