Answer:
The membrane diffusivity would be 3.968 x
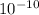
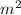 /s
/s
Step-by-step explanation:
According to Fick's law of diffusion, the rate of the purified product is related with to membrane diffusivity with the expression in equation 1.
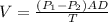 ..............................1
..............................1
Where
V is the rate of purified product = 2 kg/hr = 2 kg/hr x 1 hr / 3600 sec = 1/1800 kg/sec
 is the pressure at the supply gas point = 0.75 kg/
is the pressure at the supply gas point = 0.75 kg/
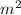
 is the pressure at the take-off side = 0.05 kg/
is the pressure at the take-off side = 0.05 kg/
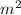
A is the area of the membrane = 100
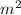
T is the thickness of the membrane = 0.05 mm = 0.05/ 1000 = 5 x
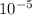 m
m
Substituting the values into equation 1 we have;
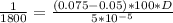
5 x
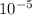 = 126000 x D
= 126000 x D
D = 5 x
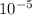 / 126000
/ 126000
D = 3.968 x
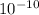
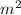 /s
/s
Therefore the membrane diffusivity would be 3.968 x
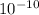
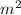 /s
/s