Answer:
![[H^+]=0.00332M](https://img.qammunity.org/2021/formulas/chemistry/high-school/brpg7hr1ozze7ael0tcnvpkcrs9zvtk7eh.png)
Step-by-step explanation:
Hello,
In this case, considering the dissociation of valeric acid as:
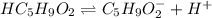
Its corresponding law of mass action is:
![Ka=([H^+][C_5H_9O_2^-])/([HC_5H_9O_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/np5mq6a0d9nblxiod88ft6dmmxouchhvpj.png)
Now, by means of the change
 due to dissociation, it becomes:
due to dissociation, it becomes:
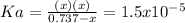
Solving for
 we obtain:
we obtain:
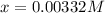
Thus, since the concentration of hydronium equals
 , the answer is:
, the answer is:
![[H^+]=x=0.00332M](https://img.qammunity.org/2021/formulas/chemistry/high-school/g3zea54k6sj8p44v7mgrlmhg22rsyrz551.png)
Best regards.