Answer:
Kp uses the same equation as Kc, but with pressure instead of concentration
Kc is equal to Kp when the number of moles of gas (n) doesn't change.
Solids and liquids are not included in the Kc and Kp equations because they have an activity equal to 1
Step-by-step explanation:
1. Kc has units of molarity and Kp has units of pressure
FALSE.
Neither Kc nor Kp has units.
2. Kp uses the same equation as Kc, but with pressure instead of concentration
TRUE.
For the reaction A(aq) ⇌ B(aq), the Kc expression is
![K_{\text{c}} = \frac{\text{[B]}}{\text{[A]}}](https://img.qammunity.org/2021/formulas/chemistry/high-school/zbqb4yswtil0pu86acu07rbh5yezgzezrp.png)
For the reaction A(g) ⇌ B(g), the Kp expression is
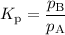
3. If there is a liquid in a reaction, you use the vapor pressure for that entry in the Kp equation.
FALSE.
If there is a liquid in a reaction, you omit it from both the Kp and Kc expressions.
4. Kc and Kp are the same thing as the k (rate constant) that you learned about before.
FALSE.
Rate constants deal with rates. Equilibrium constants deal with equilibria.
5. Kc is equal to Kp when the number of moles of gas (n) doesn't change.
TRUE
The relation between Kp and Kc is
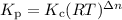
If the number of moles of gas doesn't change, Δn = 0.
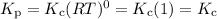
6. Solids and liquids are not included in the Kc and Kp equations because they have an activity equal to 1.
TRUE