Step-by-step explanation:
Given that,
Initial volume of tank, V = 6 L
Initial pressure, P = 2 atm
We need to find the final pressure when the air is placed in tanks that have the following volumes if there is no change in temperature and amount of gas:
(a) V' = 1 L
It is a case of Boyle's law. It says that volume is inversely proportional to the pressure at constant temperature. So,
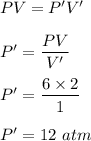
(b) V' = 2500 mL
New pressure becomes :
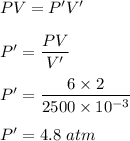
(c) V' = 750 mL
New pressure becomes :
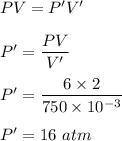
(d) V' = 8 L
New pressure becomes :
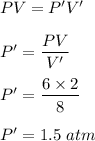
Hence, this is the required solution.