Answer:
Thus, the Quantity of
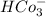 Present in urine sample in 24 hours is 35mmol
Present in urine sample in 24 hours is 35mmol
Step-by-step explanation:
Acid Production in 12 hours = 40 meq
For, 24 hours = 40 meq × 2
Acid Production in 24 hours = 80 meq
Urine sample for
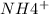 = 30mmol
= 30mmol
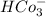 Present in Urine Sample in 24 hours
Present in Urine Sample in 24 hours
= Acid Production - Urine sample for
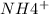 - Titratable Acid
- Titratable Acid
= 80 - 30 - 15
= 50 - 15
= 35mmol
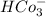 Present in urine sample in 24 hours is 35mmol
Present in urine sample in 24 hours is 35mmol