Answer:
1)
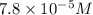 is the concentration of ergosterol in a solution.
is the concentration of ergosterol in a solution.
2) The absorbance of the solution is 0.49.
Step-by-step explanation:
Using Beer-Lambert's law :
Formula used :

where,
A = absorbance of solution
C = concentration of solution =
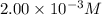
l = path length = 1.00 cm
 = molar absorptivity coefficient
= molar absorptivity coefficient
1)
We have:
Absorbance of the solution ,A = 0.93
Concentration of ergosterol ,C = ?
Path length ,l = 1.00 cm
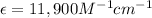

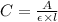
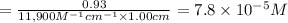
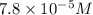 is the concentration of ergosterol in a solution.
is the concentration of ergosterol in a solution.
2)
We have:
Absorbance of the solution ,A = ?
Concentration of ergosterol ,C =
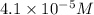
Path length ,l = 1.00 cm
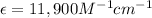

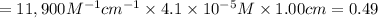
The absorbance of the solution is 0.49.