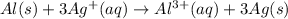Answer : The balanced two-half reactions will be,
Oxidation half reaction :
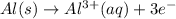
Reduction half reaction :
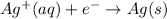
Explanation :
Voltaic cell : It is defined as a device which is used for the conversion of the chemical energy produces in a redox reaction into the electrical energy. It is also known as the galvanic cell or electrochemical cell.
The redox reaction occurs between the aluminum and silver.
In the voltaic cell, the oxidation occurs at an anode which is a negative electrode and the reduction occurs at the cathode which is a positive electrode.
The balanced two-half reactions will be,
Oxidation half reaction :
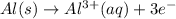
Reduction half reaction :
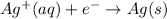
Thus the overall reaction will be,