Answer:
44 g
Step-by-step explanation:
The formula for the number of moles (n) is equal to
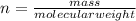 .
.
Since we need to find the mass, we derive it from the formula of the number of moles and we get that mass = n x molecular weight .
The molecular weight of
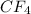 = 12 g/mol (from the carbon) + 19x4 g/mol (from the 4 fluorine atoms)= 88 g/mol
= 12 g/mol (from the carbon) + 19x4 g/mol (from the 4 fluorine atoms)= 88 g/mol
We plug in the numbers in the derived formula for the mass and we get :
mass = n x molecular weight = 0.5 mol x 88 g/mol = 44 g