Answer:
The true weight of the aluminium is
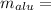 4.5021 kg
4.5021 kg
Step-by-step explanation:
Given data
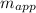 = 4.5 kg
= 4.5 kg
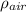 = 1.29
= 1.29
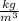
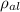 = 2.7×
= 2.7×
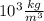
The true mass of the aluminium is given by
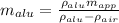
Put all the values in above equation we get
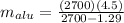
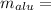 4.5021 kg
4.5021 kg
Therefore the true weight of the aluminium is
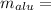 4.5021 kg
4.5021 kg