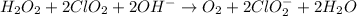Answer : The balanced chemical equation in basic medium will be,
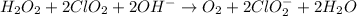
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
The given chemical reaction is,
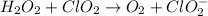
The oxidation-reduction half reaction will be :
Oxidation :
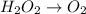
Reduction :
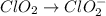
Now balance the hydrogen atom.
Oxidation :
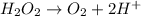
Reduction :
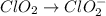
To neutralizing the hydrogen ion by adding hydroxide ion on both side.
Oxidation :
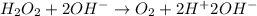
Reduction :
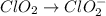
Hydrogen ion hydroxide ion combine to form water.
Oxidation :
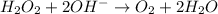
Reduction :
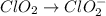
Now balance the charge.
Oxidation :
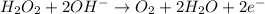
Reduction :
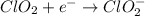
The charges are not balance on both side of the reaction. We are multiplying reduction reaction by 2 and then added both equation, we get the balanced redox reaction.
Oxidation :
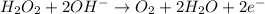
Reduction :
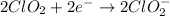
The balanced chemical equation in basic medium will be,