Answer:
(c) 18.8 g; (a) 0.798; (b) 16 mL
Step-by-step explanation:
You don't give your experimental data, so I shall assume:
Mass of Al = 1.07 g
20 mL of 3 mol·L⁻¹ KOH
20 mL of 9 mol·L⁻¹ H₂SO₄
The overall equation for the reaction is
Mᵣ: 26.98 474.39
2Al + 2KOH +4H₂SO₄ + 22H₂O ⟶ 2K[Al(SO₄)₂]·12H₂O + 3H₂
m/g: 1.07
(c) Theoretical yield of alum
(i) Moles of Al
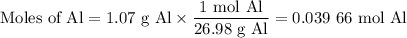
(ii) Moles of alum
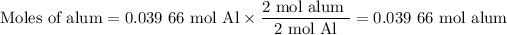
(iii) Theoretical yield of alum
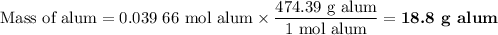
(a) Scaling factor for 15.0 g alum
You want a theoretical yield of 15.0 g, so you must scale down the reaction.
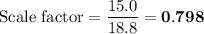
(b) Corrected volumes of NaOH and H₂SO₄
V = 0.798 × 20 mL = 16 mL