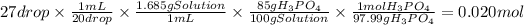Answer:
0.020 mol
Step-by-step explanation:
Let's consider the following relations:
- 20 drops of a liquid have a volume of 1 mL.
- The density of the solution is 1.685 g/mL, that is, each milliliter of solution has a mass of 1.685 g of solution.
- There are 85 g of phosphoric acid per 100 grams of solution (85 % by weight).
- The molar mass of phosphoric acid is 97.99 g/mol
With these relations, we can calculate the moles of phosphoric acid in 27 drops of solution.