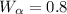Answer:
800°C
Step-by-step explanation:
Phase diagram of an alloy is a graphical presentation showing the phases compositions and their relative amounts at any different temperature and under equilibrium conditions. It is used to predict the phase changes of the alloys at different temperatures.
From the figure, we can see that the tie lines are proportional about α + L at a temperature of 800°C for