Answer:
Q = 62 MJ
Step-by-step explanation:
given data
ideal gas mixture
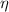 He = 3 kmol
He = 3 kmol
ideal gas mixture
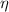 Ar = 7 kmol
Ar = 7 kmol
temperature t1 = 27°C = 300 K
pressure p1 = 200 kPa
solution
we apply here charle's law for pressure p constant that is
v ∝ T ..................1
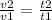
and put here value we get
\frac{2v1}{v1} = \frac{t2}{300}
solve it we get
t2 = 600 K
so here mass of He is
mass = mole of He × molecular weight He
mass = 3 × 4
mass = 12 u
and
mass of Ar is
mass = mole of Ar × molecular weight Ar
mass = 7 × 40
mass = 280
and
heat supply will be
heat supply = mHe × CpHe × ΔT + mAr × CpAr × ΔT ................2
and here we use gas table and we get
CpHe = 5.19 (kJ/(kg K)) and CpAr = 0.5203 (kJ/(kg K))
put here value and we get
Q = 12 × 5.19 × 10³ (600-300) + 280 × 0.5203 × 10³ ( 600 -300 )
Q = 62 MJ