Answer:
Yield of reaction is 76.7%
Step-by-step explanation:
The reaction of salicylic acid with acetic anhydride to produce acetyl salicylic acid is 1:1. That means 1 mole of salicylic acid reacts with 1 mole of acetic anhydride (Acid is catalyst of reaction).
Moles of salicylic acid are:
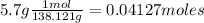
And moles of acetic anhydride are:
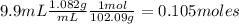
As salicylic acid is limiting reactant, theoretical moles of acetyl salicylic acid are 0.04127mol. That means theoretical mass of acetyl salicylic acid is:
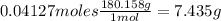
Thus, yield of reaction is:
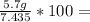 76.7%
76.7%