Answer:
The volume of nitrogen oxide formed is 35.6L
Step-by-step explanation:
The reaction of nitric acid with copper is:
Cu(s) + 4HNO₃ → Cu(NO₃)₂ + 2NO₂(g) + 2H₂O(l)
Moles of copper are:
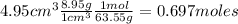
Moles of nitric acid are:
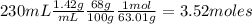
As 1 mol of Cu reacts with 4 moles of HNO₃:
0.697 mol Cu × (4mol HNO₃ / 1mol Cu) = 2.79 moles of HNO₃ will react. That means Cu is limiting reactant.
Moles of NO₂ produced are:
0.697 mol Cu × (2mol NO₂ / 1mol Cu) = 1.394 moles of NO₂
Using PV = nRT
Where P is pressure (735torr / 760 = 0.967atm); n are moles (1.394mol); R is gas constant (0.082atmL/molK); T is temperature (28.2°C + 273.15 = 301.35K).
Thus, volume is:
V = nRT / P
V = 1.394mol×0.082atmL/molK×301.35K / 0.967atm
V = 35.6L
The volume of nitrogen oxide formed is 35.6L