Answer:
27 J
Step-by-step explanation:
We are given that
Work done,w=64 J
Energy,Q=-37 J
We have to find the change in the internal energy of the air in the tank.
By First law of thermodynamics
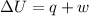
Using the formula
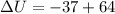
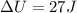
Hence, the change in the internal energy of the air in the tank=27 J