Answer: Enthalpy of combustion (per mole) of
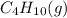 is -2657.5 kJ
is -2657.5 kJ
Step-by-step explanation:
The chemical equation for the combustion of butane follows:
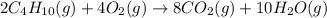
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(8* \Delta H^o_f_(CO_2(g)))+(10* \Delta H^o_f_(H_2O(g)))]-[(1* \Delta H^o_f_{C_4H_(10)(g)})+(4* \Delta H^o_f_(O_2(g)))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/zil1lzclj792dovivpmi9h5toomwaj5xbv.png)
We are given:
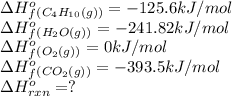
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(8* -393.5)+(10* -241.82)]-[(2* -125.6)+(4* 0)]\\\\\Delta H^o_(rxn)=-5315kJ](https://img.qammunity.org/2021/formulas/chemistry/high-school/74bb17we500m0q9ebxpgx8oep3ooxyv0vk.png)
Enthalpy of combustion (per mole) of
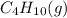 is -2657.5 kJ
is -2657.5 kJ