Pressure of the nitrogen gas is 2290 kPa.
Step-by-step explanation:
Using the ideal gas equation, we can find the pressure of the gas using the equation as,
PV = nRT
Where P is the pressure = ?
V is the volume = 3,456 ml = 3.456 L
n is the number of moles =
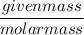 =
=
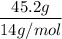 = 3.2 mol
= 3.2 mol
R is the gas constant = 0.08205 L atm K⁻¹ mol⁻¹
T is the temperature = 25 + 273 = 298 K
Now, rewriting the equation, we will get,
P =
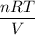
=
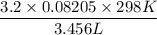
= 22.6 atm
Now Pressure in 22.6 atm is converted as 2290 kPa.