pH of 0.048 M HClO is 4.35.
Step-by-step explanation:
HClO is a weak acid and it is dissociated as,
HClO ⇄ H⁺ + ClO⁻
We can write the equilibrium expression as,
Ka =
![$([H^(+)] [ClO^(-)] )/([HClO])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mgq1nk4uygvmfh2wfa5355aodac9ybpyl6.png)
Ka = 4.0 × 10⁻⁸ M
4.0 × 10⁻⁸ M =
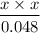
Now we can find x by rewriting the equation as,
x² = 4.0 × 10⁻⁸ × 0.048
= 1.92 × 10⁻⁹
Taking sqrt on both sides, we will get,
x = [H⁺] = 4.38 × 10⁻⁵
pH = -log₁₀[H⁺]
= - log₁₀[ 4.38 × 10⁻⁵]
= 4.35