Answer : The pressure in torr and in atmospheres are, 745 torr and 0.980 atm respectively.
Explanation :
As we are given that the atmospheric pressure is, 745 mmHg.
Now we have to determine the pressure in torr and atm.
Conversions used:
1 atm = 760 mmHg
1 atm = 760 torr
1 mmHg = 1 torr
As, 760 mmHg = 1 atm
So, 745 mmHg =
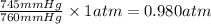
and,
As, 1 mmHg = 1 torr
So, 745 mmHg = 745 torr
Therefore, the pressure in torr and in atmospheres are, 745 torr and 0.980 atm respectively.