6.88 grams of
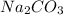 is produced from 11 grams of NaHC
is produced from 11 grams of NaHC
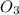 .
.
Step-by-step explanation:
Balance chemical reaction for the process:
2NaHC
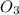 ⇒
⇒
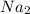
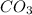 +
+
 O + C
O + C

Data given:
mass of NaHC
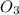 given = 11 gram
given = 11 gram
atomic mass of NaHC
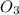 = 84.007 grams/mole
= 84.007 grams/mole
mass of
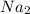
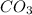 produced = ?
produced = ?
number of moles of NaHC
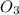 will be calculated as:
will be calculated as:
number of moles =

putting the values in the equation:
number of moles =
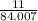
= 0.13 moles
from the balanced equation it can be seen that,
2 moles of NaHC
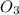 reacted to give 1 mole of
reacted to give 1 mole of
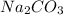
0.13 moles of NaHC
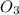 will give x moles of
will give x moles of
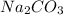
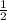 =
=
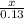
x =
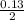
= 0.065 moles of
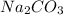
Atomic mass of
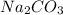 = 105.98 grams/mole
= 105.98 grams/mole
mass = atomic mass x number of moles
mass = 0.065 x 105.98
mass = 6.88 grams
so, from 11 grams of NaH
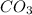 6.88 grams of
6.88 grams of
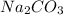 is produced.
is produced.